The Chemistry of Acid Rain and Ozone Layer Depletion
Introduction
Have you ever heard about acid rain or the ozone hole? These are not just big science terms—they are real problems affecting our air, our health, and our planet. But don’t worry! You don’t need to be a scientist to understand them.In this topic, we’ll explain:
- What acid rain and ozone layer depletion are
- Why they happen (with easy chemistry)
- How they hurt the Earth
- And most importantly—how we can stop them
Let’s dive in 🌊
What is Acid Rain?
Simple Definition:
Acid rain is rain that has more acid in it than normal. This happens when polluted air mixes with clouds and makes the rain water sour like lemon juice!
What Causes Acid Rain?
The biggest cause of acid rain is air pollution from:
- Factories
- Power plants
- Cars and trucks
These machines burn fossil fuels like coal, oil, and gas. When this happens, they release harmful gases:
- Sulfur dioxide (SO₂)
- Nitrogen oxides (NOₓ)
These gases float into the sky and mix with water droplets in clouds.
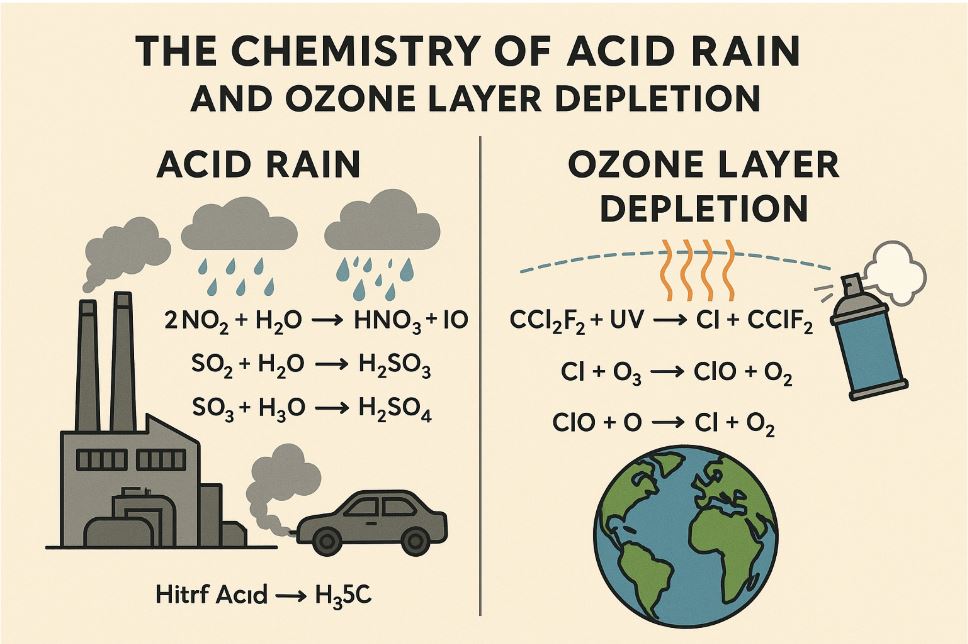
How Acid Rain Forms – The Chemistry
Let’s make it simple:
| Pollutant Gas | What It Becomes | Final Result |
|---|---|---|
| SO₂ (sulfur dioxide) | Mixes with oxygen and water | Turns into sulfuric acid (H₂SO₄) |
| NOₓ (nitrogen oxides) | Mixes with water | Forms nitric acid (HNO₃) |
When it rains, the water contains these acids—and that’s acid rain.
Effects of Acid Rain
| Area Affected | What Happens |
|---|---|
| 🌲 Forests | Trees get sick and lose their leaves |
| 🐟 Lakes | Fish die due to acid in water |
| 🏛️ Buildings | Statues and monuments get damaged |
| 🌱 Soil | Plants don’t grow well because nutrients get washed away |
How Can We Stop Acid Rain?
- Use clean energy like wind or solar
- Ride a bike or take a bus instead of using cars
- Install filters in factories to clean the smoke
- Recycle and reduce waste
What is the Ozone Layer?
Simple Definition:
The ozone layer is a part of the sky that protects us from the Sun’s harmful rays. Without it, the Sun could harm our skin, eyes, and the environment.
Where Is the Ozone Layer?
- It lives in the stratosphere, one of the layers of the sky
- It’s about 15 to 35 kilometers above Earth
- It's made of a special gas called ozone (O₃)
What Destroys the Ozone Layer?
The main villain here is a group of chemicals called CFCs (chlorofluorocarbons).They were used in:
- Old refrigerators
- Spray cans
- Air conditioners
These CFCs go up into the sky and break down ozone.
How Ozone Gets Destroyed – The Chemistry
| Step | What Happens |
|---|---|
| 1 | CFC breaks apart and releases chlorine (Cl•) |
| 2 | Cl• meets ozone (O₃) and destroys it |
| 3 | The same chlorine atom keeps destroying more ozone |
That’s why even a tiny amount of CFCs can do big damage!
Effects of Ozone Layer Depletion
| Problem | What Happens |
|---|---|
| 😖 More UV rays | Can cause skin cancer and sunburn |
| 👁️ Eye damage | Like cataracts |
| 🐠 Ocean life | Harm to small sea animals (plankton) |
| 🌾 Farming | Crops grow slower or get damaged |
How Can We Protect the Ozone Layer?
- Stop using old sprays and fridges with CFCs
- Buy ozone-friendly products
- Support the Montreal Protocol (a global agreement to ban ozone-harming chemicals)
- Spread awareness and teach others!
Quick Comparison: Acid Rain vs Ozone Layer Depletion
| Feature | Acid Rain | Ozone Depletion |
|---|---|---|
| Main Cause | SO₂ and NOₓ | CFCs (chlorofluorocarbons) |
| Affects | Earth's surface | Upper atmosphere (stratosphere) |
| Main Problem | Acid in rain damages nature | Sun’s UV rays reach Earth |
| Chemistry Involves | Formation of acids | Destruction of ozone (O₃) |
| Can We Fix It? | Yes! With clean energy | Yes! By banning CFCs |
Final Thoughts: Why This Matters
Both acid rain and ozone depletion are caused by pollution. The good news is—we can understand the science and take action to fix them.Even small steps like using eco-friendly products, saving energy, and learning about the environment can help protect the Earth.